Trusted Services
Since 2005
Trusted Services
24/7 Support
Since 2005
We Have Technologies For
WATER QUALITY | PIPED WATER SUPPLY | FOOD WASTE | PACKAGED STP
OUR STORY
About Us
HES Water Engineers (India) Pvt. Ltd. is a pioneer in designing and manufacturing innovative & technologically advanced water purification systems, wastewater treatment equipment, food waste management systems, among others.
Established in the year 2005 at Nagpur, in one of India’s largest and top-most industrial estates M.I.D.C. Butibori, we are committed to providing our clients with a spectrum of world-class products that help resolve various issues related to drinking water, industrial water, wastewater, and solid waste management. We have also worked on water distribution systems for Rural Piped Water Supply Schemes under the Government of India’s Jal Jeevan Mission.

OUR
Business Segment

Drinking Water Treatment Solutions

Industrial Water Treatment Solutions

Water Distribution - Rural Piped Water Supply Schemes - JAL JEEVAN MISSION (JJM)
OUR
Business Segment

Drinking Water Treatment Solutions
Industrial Water Treatment Solutions

Water Distribution - Rural Piped Water Supply Schemes - JAL JEEVAN MISSION (JJM)
Sewage Treatment Plants

Food and Organic Waste Management
Interested In our Products?
Interested In our Products?
OUR
Products

CSIR AMPRI Handheld Hypochlorite Generator
AMPRICARE: Instantaneous hypochlorite generator using kitchen salt…


OxiMax Community Clear Drinking Water System (RO and UF)
Since almost all drinking water sources…

Sewage Treatment Plant
To help save water and the environment, we at HES Water Engineers India…
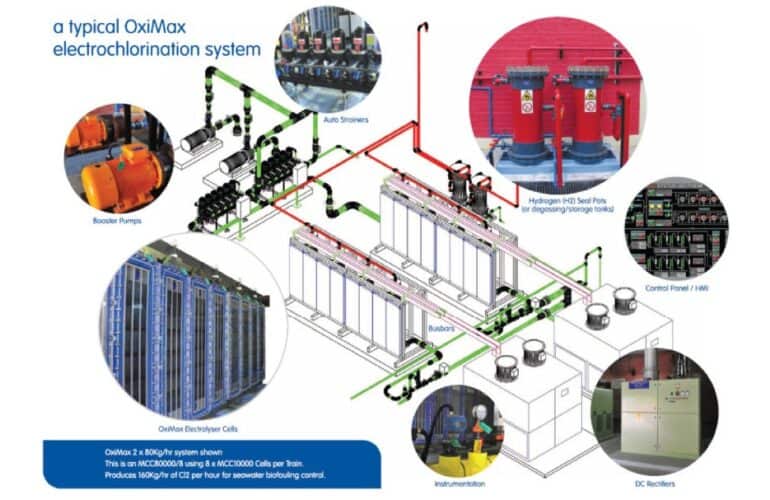
Electrochlorination Systems
We at HES Water Engineers India Pvt. Ltd. are a leading manufacturer…

CSIR-NEERI OxiMax Electrolytic Defluoridation (EDF) System
It is estimated that in India, 62 million people including 6 million…

OUR
Products

CSIR AMPRI Handheld Hypochlorite Generator
AMPRICARE: Instantaneous hypochlorite generator using kitchen salt…


OxiMax Community Clear Drinking Water System (RO and UF)
Since almost all drinking water sources…

Sewage Treatment Plant
To help save water and the environment, we at HES Water Engineers India…

Electrochlorination Systems
We at HES Water Engineers India Pvt. Ltd. are a leading manufacturer…

CSIR-NEERI OxiMax Electrolytic Defluoridation (EDF) System
It is estimated that in India, 62 million people including 6 million…

10000 +
Plants Installed
NUMBER SPEAKS
We are always ready
for a challenge.
18 +
States of India Covered
10000 +
Community Water Treatment Plants
Govt of West Bengal

Govt of Tamil Nadu

Govt of Jammu & Kashmir

PHED Rajasthan

Govt of Rajasthan

Govt of Kerala

Govt of Odisha

PHED Uttar Pradesh

Govt of Uttar Pradesh

Govt of Tripura

Wsso

MJP Maharashtra

Govt of Maharshtra

PHED Madhya Pradesh

Govt Madhya Pradesh

KUWSDB Karnataka

Govt of Karnataka

RWSD Jharkhand New

RWSD_Jharkhand

Govt of Hariyana

GWSSB Gujarat

Govt of Gujarat

PHED Chhattisgarh

PHED-Bihar-Recruitment

PHED Bihar State

Govt of Andhra Pradesh

Neeri

CSIRCMERI

Coal India

NCC

Paradip port Trust

ONGC

AIIMS

Water Aid

Reliance

Jusco

Vedanta

NEERI

Xylem

Anarde

NPCIL

NHDC

Indian Oil

Tata Motors Limited

Indian Railways

Punj Lloyd

IDCO

NSPCL

APGENCO

Technology
Partners
Bluact Technologies

Neeri

CSIR AMPRI

CSIRCMERI





